Dr. Kevin Lewis
Professor
|
Contact Information Office: CHEM 217 Phone: (512) 245-8594 Fax: (512) 245-2374 email: ll18@txstate.edu |
Educational Background
|
Areas of InterestAnalysis of genes and proteins required for repair of DNA that has been damaged by radiation or chemicals; importance of DNA repair in aging; development of new techniques for purification and analysis of DNA and RNA; study of interactions between nucleic acids and nanomaterials with potential for use in gene delivery into cells. |
|
Related Web SitesLewis Research Group Webpage |
Research in the Lewis Group
The chromosomes within animal and plant cells are continually engaged in a cycle of DNA damage and repair. One common type of damage involves breakage of one or both of the long strands of the DNA double helix. This type of DNA damage is particularly prevalent after exposure of cells to agents such as X-rays or radon gas and is also induced by free radicals and by many chemicals used in cancer chemotherapy.
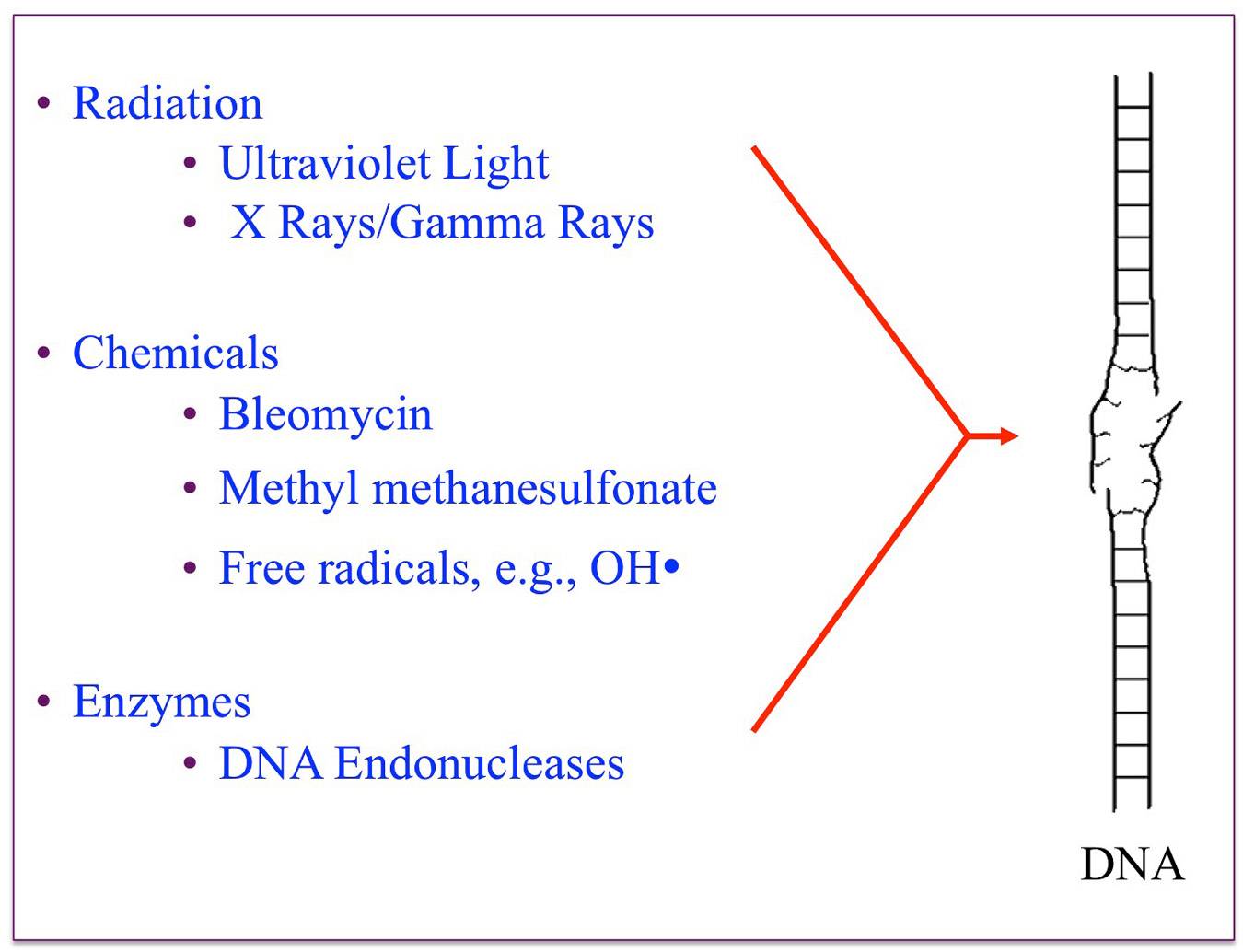
Restoration of the broken DNA double helix requires the coordinated activities of a large number of cellular proteins. The laboratory is applying modern techniques of molecular biology and biochemistry to identify and characterize genes involved in repair of strand breaks and other types of DNA damage. Several genes identified in this work have been found to affect other aspects of DNA metabolism, including cell cycle checkpoint responses and telomere stability. Furthermore, a subset of these genes encode proteins homologous to human proteins previously implicated in a number of human diseases, including cancer. Understanding these processes and the multiple pathways involved in DNA strand break repair are major goals of current research in the lab.
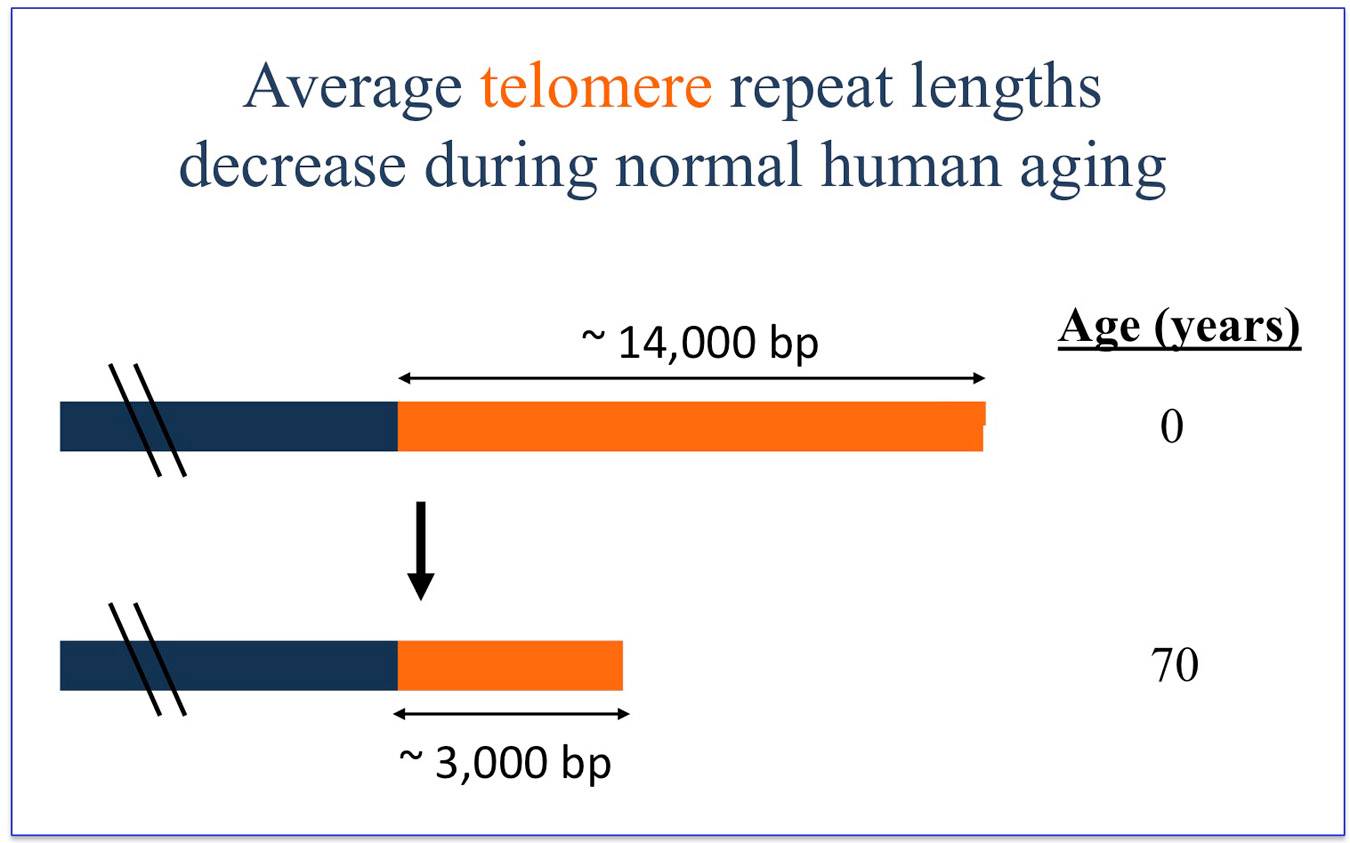
A second major effort in the lab involves the study of cellular aging. Most human cells stop producing telomerase, a DNA polymerase enzyme, early in development. Both in vivo and when grown in culture, the telomerase-deficient cells undergo progressive shortening at the ends of their chromosomes, at regions called telomeres. A new system developed in the lab allows modulated expression of telomerase inside cells. This expression system has been used to perform critical tests of current models for cell aging and cell death.
Recent Publications
Shubha R. L. Malla†, Archana Gujjari†, Carlos E. Corona‡, Gary W. Beall and L. Kevin Lewis*. 2023. Spectrophotometric and nucleic acid-binding properties of halloysite clay nanotubes and kaolinite. Heliyon 9:e13009.
Cory L. Holland, Monica F. Weis†, Corbin J. England†, Armand M. Berry†, Paige Hall‡ and L. Kevin Lewis*. 2023. Deficiency in homologous recombination is associated with changes in cell cycling and morphology in Saccharomyces cerevisiae. Experimental Cell Research 430:113701.
O’Taveon R. Fitzgerald†, Nestor D. Rodriguez† and L. Kevin Lewis*. 2022. High efficiency plasmid DNA transformation in yeast. Invited paper: Methods in Molecular Biology. 2513:15-22.
Cory L. Holland, Brian A. Sanderson†, James K. Titus†, Monica F. Weis†, Angelica M. Riojas†, Eric Malczewskyj†, Brian M. Wasko and L. Kevin Lewis*. 2021. Role of DNA repair and replication factors in the suppression of telomere instability in Saccharomyces cerevisiaeyku70 mutants. Genes, Genomes & Genetics 11:jkab359.
Corbin J. England†, Tanner C. Gray‡, Shubha R. L. Malla†, Samantha A. Oliveira‡, Gary W. Beall and L. Kevin Lewis*. 2020. pH-dependent sedimentation of DNA in the presence of divalent, but not monovalent, metal ions. Analytical Biochemistry 616:114099.
Neda Z. Ghanem†, Shubha R. L. Malla†, Naoko Araki† and L. Kevin Lewis*. 2019. Quantitative assessment of changes in cell growth, size and morphology during telomere-initiated cellular senescence in Saccharomyces cerevisiae. 2019. Experimental Cell Research 381:18-28.
Jennifer A. Ream‡, L. Kevin Lewis and Karen A. Lewis. 2018. Horizontal agarose gel mobility shift assay for protein-RNA complexes. Methods in Molecular Biology 1855:363-370. Published online 11-14-18.
Archana Gujjari†, Blanca V. Rodriguez†, Jorge Pescador‡, Corina Maeder, Gary W. Beall and L. Kevin Lewis*. 2017. Factors affecting the association of single- and double-stranded RNAs with montomorillonite nanoclays. International Journal of Biological Macromolecules 109:551-559. Epub Dec 22, 2017.
Whitney N. Wood‡, Kyle D. Smith‡, Jennifer A. Ream‡, and L. Kevin Lewis*. 2016. Enhancing yields of low and single copy number plasmid DNAs from Escherichia coli cells. Journal of Microbiological Methods. 133:46-51. Published online on 12-23-16. DOI: 10.1016/j.mimet.2016.12.016
Jennifer A. Ream‡, L. Kevin Lewis and Karen A. Lewis. 2016. Rapid agarose gel electrophoretic mobility shift assay for quantitating protein:RNA interactions. Analytical Biochemistry 511:36-41.
Blanca V. Rodriguez†, Jorge Pescador‡, Nicole Pollok‡, Gary W. Beall, Corina Maeder and L. Kevin Lewis*. 2015. Impact of size, secondary structure and counterions on the binding of small RNAs to layered double hydroxide nanoparticles. Biointerphases 10(4):041007.
Blanca V. Rodriguez†, Eric T. Malczewskyj†, Joshua M. Cabiya‡, L. Kevin Lewis and Corina Maeder. 2015. Identification of an RNase-resistant population of RNAs in Saccharomyces cerevisiae extracts: separation from chromosomal DNA by selective precipitation. Analytical Biochemistry 492:69-75. (Epub 2015 Sep 28)
Brian A. Sanderson†, Naoko Araki†, Jennifer L. Lilley‡, Gilberto Guerrero‡ and L. Kevin Lewis*. 2014. Modification of gel architecture and TBE/TAE buffer composition to minimize heating during agarose gel electrophoresis. Analytical Biochemistry 454:44-52.
Jennifer Summers McKinney†, Sunaina Sethi†, Jennifer DeMars Tripp†, Thuy N. Nguyen†, Brian A. Sanderson†, James W. Westmoreland, Michael A. Resnick and L. Kevin Lewis*. 2013. A multistep genomic screen identifies new genes required for repair of DNA double-strand breaks in Saccharomyces cerevisiae. BMC Genomics 14:251-266.
Jennifer DeMars Tripp†, Jennifer L. Lilley‡, Whitney N. Wood‡ and L. Kevin Lewis*. 2013. Enhancement of plasmid DNA transformation efficiencies in early stationary phase yeast cell cultures. Yeast 30:191-200.

